Author: |
The development of efficient electrocatalyst to produce molecular hydrogen from water is receiving considerable attention, in an effort to decrease our reliance on fossil fuels. Recently, silver sulfide (Ag2S) nanocrystals have attracted enormous interests due to its excellent properties, including negligible toxicity, adjustable band gap through size control and surface modification and long term stability in acid environment. At present, the performance of hydrogen generation reaction (HER) catalysts based on Ag2S nanocrystals are still at a certain distance from expectations. One of the main reasons is that the impeded charge transfer and decreased active sites caused by aggregation of Ag2S nanocrystals. Many research groups have combined the Ag2S with other electron transfer materials to improve the catalysts performance and have achieved certain results. But the negative effects of aggregation still needs to be solved.
In order to solve this problem, the team of scientists, led by Prof. YU Weili from Changchun Institute of Optics, Fine Mechanics, and Physics (CIOMP) of the Chinese Academy of Sciences and Prof. Hicham Idriss of King Abdullah University of Science and Technology (KAUST) successfully synthesized Ag2S/rGO composite catalysts with smaller crystal size and better charge transfer properties by the solution fabrication strategy. The high quality Ag2S nanocrystals were combined with reduced graphene oxide with high carrier mobility, an efficient HER catalysts were prepared.
HER catalysts made with these Ag2S/rGO composites show excellent catalytic performance with a low Tafel slope (≈49.1 mV/dec) and a low overpotential (≈120 mV) at a current density (10 mA/cm2) in 0.5 M H2SO4 Aqueous solution. Compared to catalyst based on pure Ag2S nanocrystals, the Ag2S/rGO composites catalyst showed a significant decrease of overpotential, Tafel slope and electrochemical resistance. Transmission electron microscope (TEM) images show that the induced rGO provides abundant nucleation sites thus prevents the aggregation of Ag2S nanocrystals. The average size of Ag2S nanocrystals grown on rGO is calculated to be about 7 nm. Time-resolved photoluminescence (TRPL) studies show that the improvement of the catalystic performance is mainly attributed to the efficient charge transfer of the Ag2S/rGO composites.
In this study, Ag2S nanocrystals and two-dimensional material rGO are effectively combined to achieve a significant improvement in catalytic performance by taking advantage of the synergistic advantages of the two materials in carrier transfer and aggregation inhibition. This demonstrates the ability of catalysts structure and composition design to improve the performance of the catalysts, and reveals the charge transfer and aggregation inhibition, which provides a new way for the preparation of high performance composite HER catalysts. The relevant results are published on Catalysts (DOI: 10.3390/catal10090948) under the title “Effect of Ag2S Nanocrystals/Reduced Graphene Oxide Interface on Hydrogen Evolution Reaction”
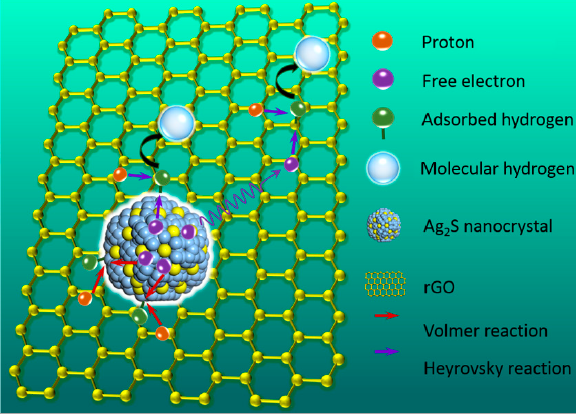
Schematic of the HER reaction catalyzed by Ag2S/rGO (Photo by CIOMP)
Author: YU Weili
E-mail: weili.yu@ciomp.ac.cn
Article links: https://www.mdpi.com/2073-4344/10/9/948
